Describe How Acid Rain Would React With Rocks Over Time
Acid rains ability to dissolve marble and limestone makes it hazardous to buildings and outdoor monuments. Acid rain slowly dissolves rocks due to chemical reactions between the acid and the minerals in the rock.
How Does Acid Rain Affect The Rocks Over Hundreds Of Years Quora
Solution weathering also covers other types of chemical solutions such as basic rather than acidic ones.
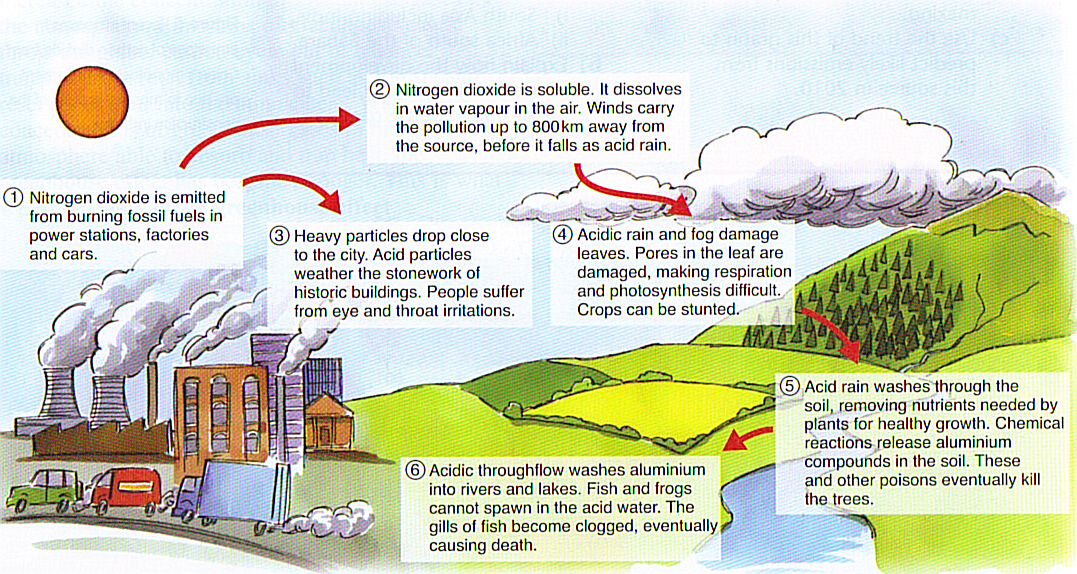
. Acid rain results when sulfur dioxide SO 2 and nitrogen oxides NO X are emitted into the atmosphere and transported by wind and air currentsThe SO 2 and NO X react with water oxygen and other chemicals to form sulfuric and nitric acids. However carbonate rocks such as limestone and marble can be noticeably affected by acid rain because they predominantly contain calcium carbonate which is far more reactive to acid attack than. First the strength of the acid.
Permalink Submitted by chris on Sun 05112017 - 1022. As the limestone dissolves the rocks will wear away becoming pitted with rounded edges. - steep sided mountains will increase rockland sides exposing fresh surfaces as well as forcing clouds upslope and.
Acid rain is caused by the omission of harmful particles into the atmosphere including sulfur dioxide SO 2 and nitrogen oxide NO 2Acid rain can have harmful effects on plants marine animals humans infrastructure and more. The acidic water kills aquatic and organisms like planktons and fishes disturbing ecosystem. Calcium-based minerals such as marble and limestone are particularly vulnerable.
The effect of acids on minerals is an example of solution weathering. The dissolution process will change the appearance of the landscape as the rocks slowly wear away over time. Acid rain is rain with an unusually low pH level meaning it is extremely acidic.
Although the lemon juice and vinegar acids used in this activity are more concentrated than acid rain they successfully demonstrate the erosive effects of acid rain over time. Igneous and metamorphic rocks exposed to acid rain can. When rocks and minerals are altered by hydrolysis acids may be produced.
Through hydrolysis reactions minerals are weathered in the presence of water. Acid rain slowly dissolves many types of stone. Rainwater is naturally slightly acidic because carbon dioxide from the air dissolves in it.
Acid rain has the following reaction with the marble calcium carbonate. The weathering of rocks by chemicals is called chemical weathering. Fes H 2 SO 4 aq FeSO 4 aq H 2 g CaCO 3 s H 2 SO 4 aq CaSO 4 s CO 2 g H 2 Ol Water pollution.
Limestone is calcium carbonate CaCO 3. Carbonates react with acids according to the equation. Yes limestone reacts with acids.
Describe how acid rain would react with rocks over time. Minerals in rocks may react with the rainwater causing the rock to be weathered. Are made of a mineral called calcium carbonate and.
When acidic rainwater falls on limestone or chalk a chemical reaction happens. Softer less resistant rocks wear away at a faster rate than more weather resistant rocks. Weathering from Acid Rain.
In exposed areas of buildings and statues we see roughened surfaces removal of material and loss of carved details. Correct answer to the question Describe how acid rain would react with rocks over time. However the rate of the reaction will be determined by a number of factors.
The effect of acid rain on most rocks is insignificant since the majority of rocks making up the Earths crust 92 are silicate-based and dont readily react with even strong acids. Three types of chemical weathering processes are particularly important for transforming minerals within rocks into weathered products. When sulfurous sulfuric and nitric acids in polluted air and rain react with the calcite in marble and limestone the calcite dissolves.
Hydrolysis takes place when acid rain reacts with rock-forming minerals such as feldspar to produce soluble. New soluble substances are formed in the reaction. HOPE IT HELPS PLEASE MARK IT THE BRAINLIEST.
While a small portion of the SO 2 and NO X that cause. - long period of time - activity of rock weathering by acid rain is viewed as the active driver of climate change in the uplift weathering hypothesis - continental collisions produce mountains and plateaus which greatly increase fresh rock surface area to be weathered. Metals like iron and calcium carbonate react with the acid in the rain slowly as follows.
These are hydrolysis oxidation and dissolution. As acid rain falls to the earths surface limestone rocks and limestone components in soil will react with the rain neutralize the acid and dissolve. Dissolves rain water and forms carbonic acid which easily weathers rocks such as Marble and Limestone living organisms Plants have roots that naturally produce weak acids when they grow slowly dissolving rock around roots.
The acid causes the calcium Ca and carbonate CO 3 in the limestone to separate into calcium and carbon dioxide gas CO 2. This is due to calciums reaction with the sulfuric acid in acid rain. CO 32- 2H - H 2 O CO 2.
When the acid rain touches the rock it starts to break down the molecules in the rock causing cracks to appear leading to bits falling of. CaCO 3 H 2 SO 4 CaSO 4 H 2 O CO 2 The formation of calcium sulphate. Stone surface material may be lost all over or only in spots that are more reactive.
These then mix with water and other materials before falling to the ground. Acid rain increases the acidity in rivers and lakes. These dissolve in the water and then are washed away.
Acids may also be produced when water reacts with the atmosphere so acidic water can react with rocks.

Ess Topic 6 4 Acid Deposition Amazing World Of Science With Mr Green

Acid Rain Formation Effects And Control Measure Forestrypedia
0 Response to "Describe How Acid Rain Would React With Rocks Over Time"
Post a Comment